 ALERT
ALERT **Important for iLab Users**
The Help link in iLab opens an email to contact Support. To submit or track requests, visit: Submit and Track Support Requests
Flow cytometry is a central technology in biomedical research allowing multiparametric analysis and isolation (by cell sorting) of rare cell populations down to a single cell from complex cell mixtures.
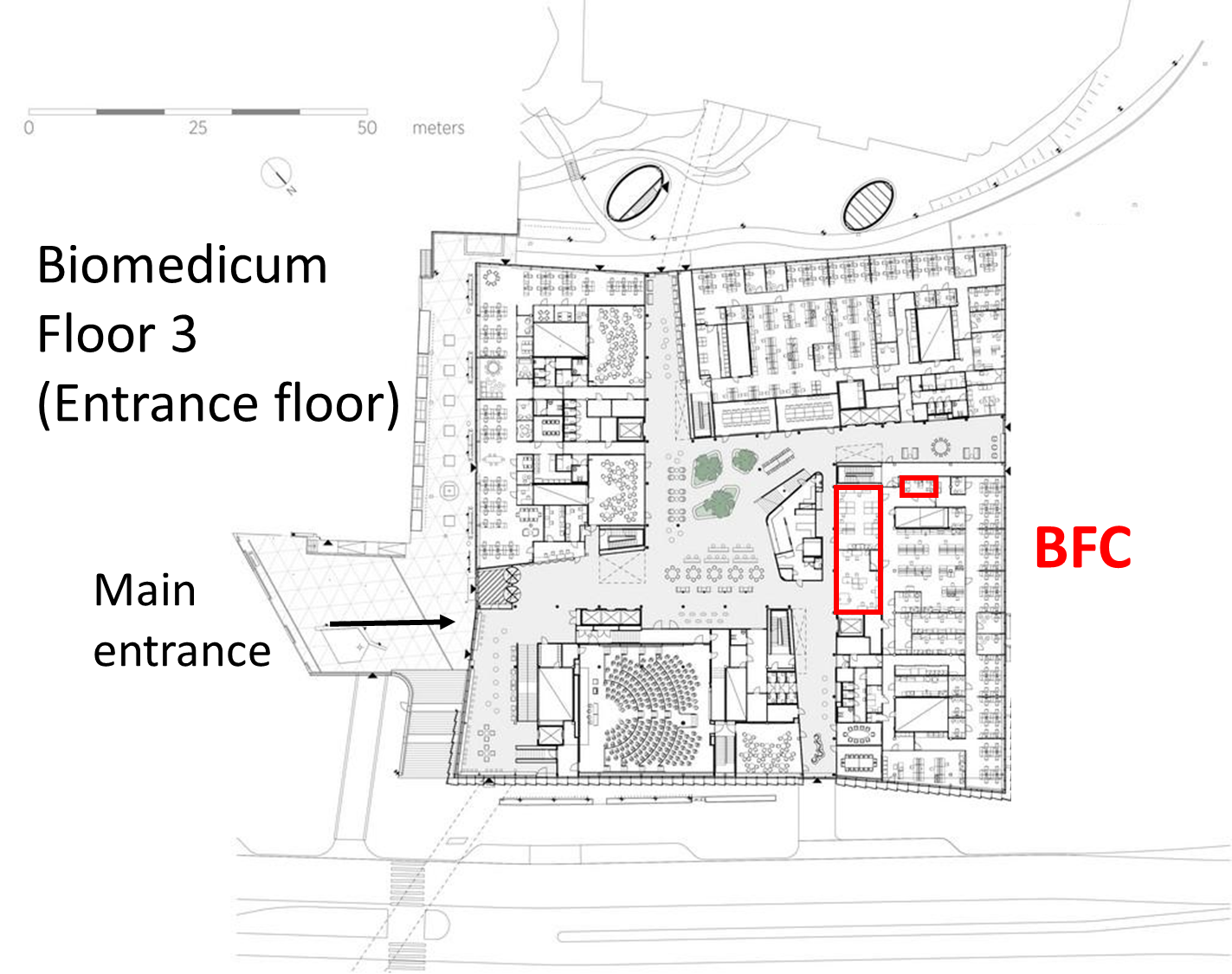
The Biomedicum Flow cytometry Core facility (BFC) is located at the 3rd (entrance) floor of the Biomedicum research building at the Karolinska Solna Campus (see visiting address). The BFC provides open access to all interested researchers at KI and Karolinska Hospital regardless of their affiliation and strives to provide each user with skilled services, education and training to achieve high quality research.
The facility was first established in the 1980s at Tumor Biology department. It belongs to the department MTC and has received support from Knut och Alice Wallenbergs Stiftelse, it is currently supported by core facility funding from KI/SLL. To cover running costs, the facility charges fees for services (see user Fees for detailed information).
When publishing data generated from the facility, please use the following phrase: “We acknowledge the Biomedicum Flow cytometry Core facility (Karolinska Institutet), supported by KI/SLL, for providing cell sorting services**/cell analysis services**/technical expertise**/scientific input**”. **delete as required.
A risk assessment shall be carried out in order that everyone shall have a safe work environment, and to raise risk awareness. Prior to cell sorting, the facility requires individual risk assessment for each new project (under the "Request Servies")
| Hours | Location |
|
Monday - Friday 9:00-17:00h |
Biomedicum floor 3 (C3) Solnavägen 9, SE-17177 Stockholm |
BFC FACS website
A more detailed introduction to flow cytometry
Training for using DiVa software
More Flow Cytometry Links:
http://www.cyto.purdue.edu/hmarchiv/index.htm
http://www.bdbiosciences.com/us/s/spectrumviewer?cc=US
http://www.biolegend.com/spectraanalyzer
| Name | Role | Phone | Location | |
|---|---|---|---|---|
| Maria Johansson |
Facility Manager
|
08-52486739, 0730 386452
|
maria.johansson@ki.se
|
Biomedicum C0312
|